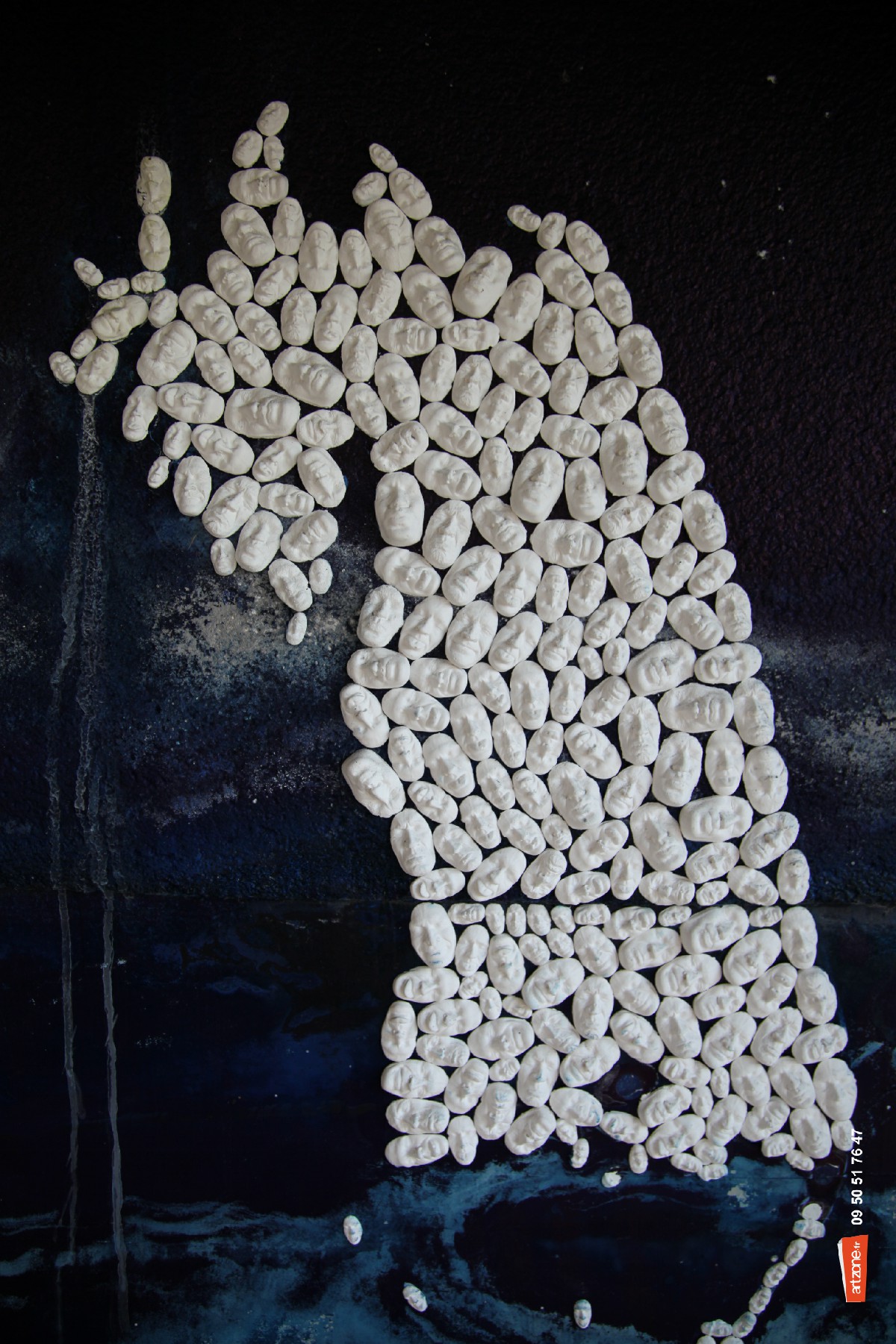
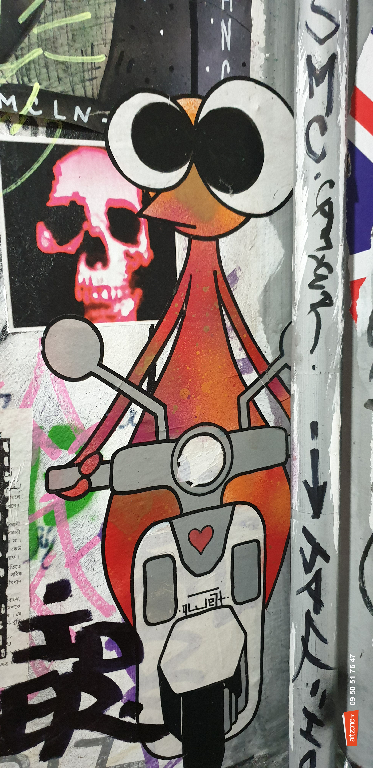
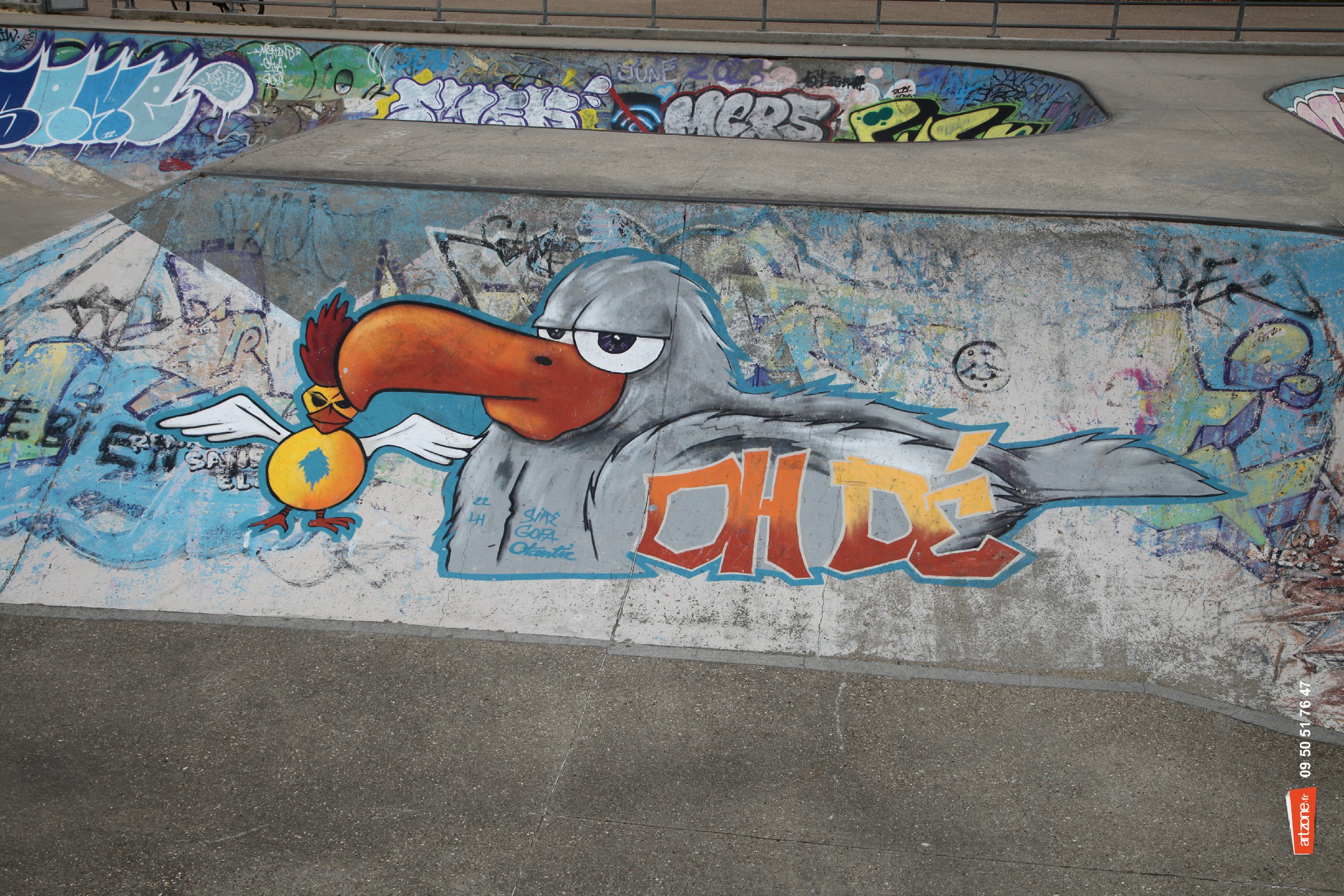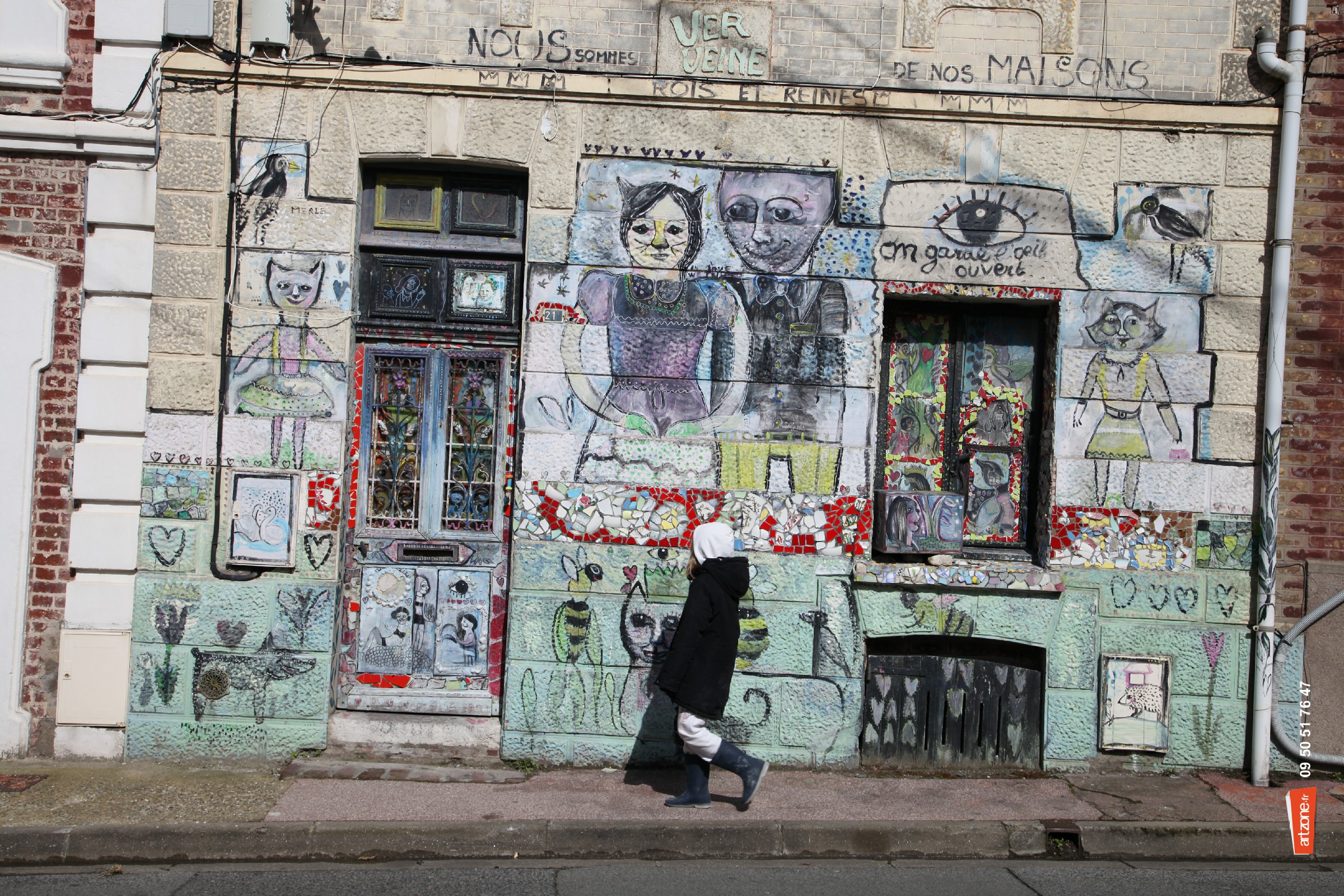Photographiés en plein jour
Que deviennent-ils la nuit ?
Foules paillettes alentours
Mille visages sans un bruit
De leur maison de parpaings
De pierre, de brique et d’acier
Ils tapinent dans un coin
Dans leur cages en 2 D
Vous qui passez bien tranquille
Ils vous observent bizarres
Est-ce que vos yeux s’écarquillent ?
Est-ce le fruit du hasard ?